Pioneering a New Class of Vaccines for Global Unmet Medical Needs
Constantly-Mutating Global Infectious Diseases
Game-Changing Technology
Diversified pipeline
Multiple combination opportunities
The oligoDOMTM technology platform can also be combined with other innovative vaccine technologies, like adjuvants and mRNA. Osivax is currently investigating the potential synergies of such combinations.
Our Pipeline
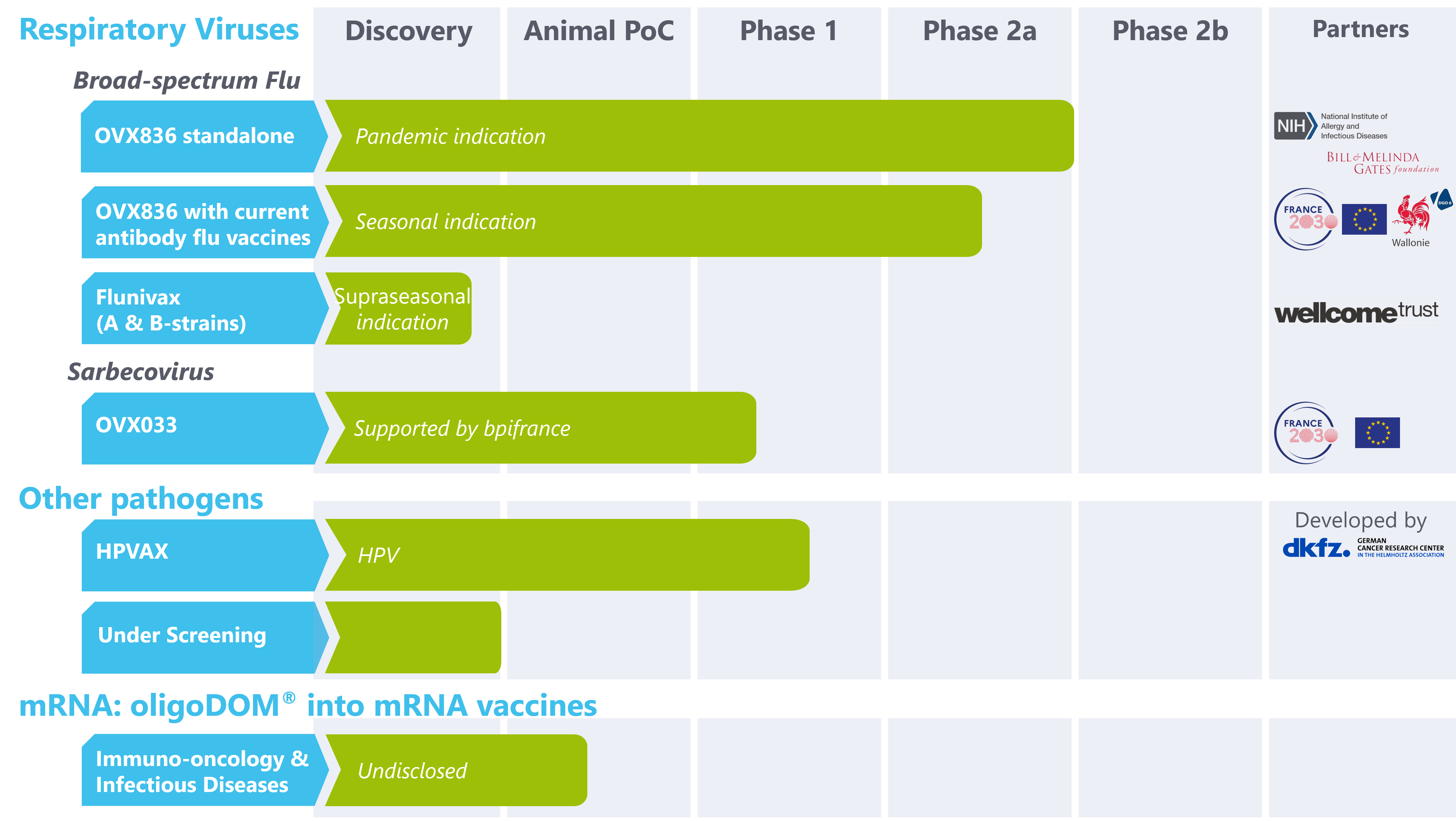
HPV: Human PapillomaVirus
POC: Proof-Of-Concept
OVX836
Universal Flu
Vaccine Candidate
OVX033
Universal Sarbecovirus
Vaccine Candidate
OVX836 – Universal Flu Vaccine Candidate
Facts about the
seasonal influenza
Influenza is a devastating disease, with routine global outbreaks that pose a profound threat to global health. Seasonal influenza kills hundreds of thousands of people annually and pandemic influenza can cause tens of millions of deaths as demonstrated by historic outbreaks.
Effectiveness
by flu every season worldwide
emergence of a pandemic strain
within 6 months of time.
Current seasonal flu vaccines are only partially effective because they target strain-specific surface antigens, which mutate at high rates and thus change seasonally. A universal vaccine presents the highly needed opportunity to provide broad coverage across all seasonal and pandemic strains.
Osivax is creating a revolutionary universal influenza vaccine which will uniquely empower both the B-cell immune response and the T-cell immune response. Osivax will combine its best-in-class T-cell component (OVX836) with conventional flu vaccines to fully empower both arms of the immune system.
OVX836 targets the nucleoprotein (NP), a highly conserved internal antigen. Unlike surface antigens, the NP is much less likely to mutate, alleviating the need for annual vaccination updates. Osivax’ oligoDOMTM technology enables the transformation of the NP into a highly immunogenic antigen to trigger powerful T-cell immune responses.
Osivax’ lead candidate has shown powerful and long-lasting systemic and local immune responses in preclinical development. In vivo, it has shown strong efficacy against both A and B strain flu viruses and an excellent safety profile in a GLP toxicology study. After successful Phase 1 and multiple Phase 2a clinical trials, OVX836 is currently being evaluated in a booster trial in Belgium.
OVX033 – Universal Sarbecovirus Vaccine Candidate
Facts about the
Coronavirus
over the past 20 years
Currently commercialized vaccines target the constantly evolving spike surface antigens with the aim to generate B-cell responses. Osivax is creating a revolutionary universal sarbecovirus vaccine which will uniquely empower both the B-cell immune response and the T-cell immune response.
Osivax is engineering a vaccine (OVX033) that targets the virus’ nucleocapsid, an internal and invariant antigen. This target can be transformed into a highly immunogenic antigen when enabled by the oligoDOMTM technology and is expected to provide universal breadth of protection against all current and future sarbecoviruses.
Osivax builds on the experience gained through the development of OVX836 in influenza which facilitates the development and preclinical proof-of-concept of OVX033 in less than one year. OVX033 is being evaluated in a First-In-Human trial.
This project has received funding from the European Union’s Horizon 2020 research and innovation program under grant agreement No 961112. This project is supported by the French State, through the Banque Publique d’Investissement (BPI) France’s program: Programme d’investissements d’avenir and France 2030.





