
oligoDOMTM: A Novel Proprietary Nanoparticles Technology specifically designed to trigger superior T-cell responses, in addition to strong and sustained B-cell responses
The core concept
Nanoparticles self-assemble to present key immunogenic structures of a specific virus in repetition to induce an immune response.
Improving and surpassing the Nanoparticles technology
Building upon the established Nanoparticles concept
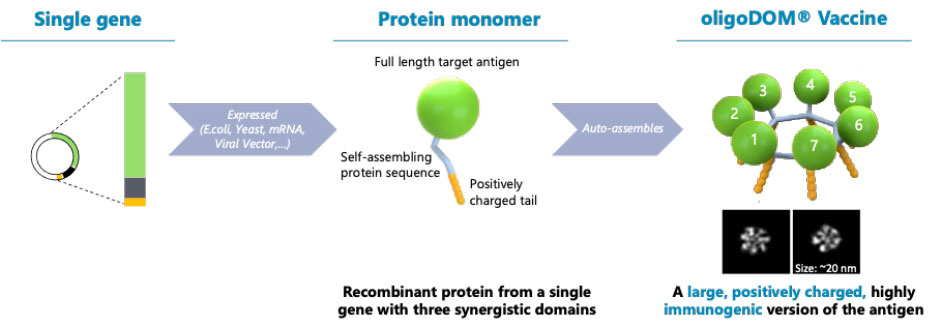
Given its design and mechanism of action, the oligoDOMTM technology platform is highly versatile and can be used with different antigens.
Osivax, together with its partners, is developing several vaccine candidates, 3 of which are already in clinical phase.
As of today the oligoDOMTM technology has been evaluated in 7 clinical trials with more than 1,600 subjects.
Osivax will expand its portfolio in other areas through collaborations and partnerships.
Preventing the Spread of constantly mutating Global Infectious Diseases
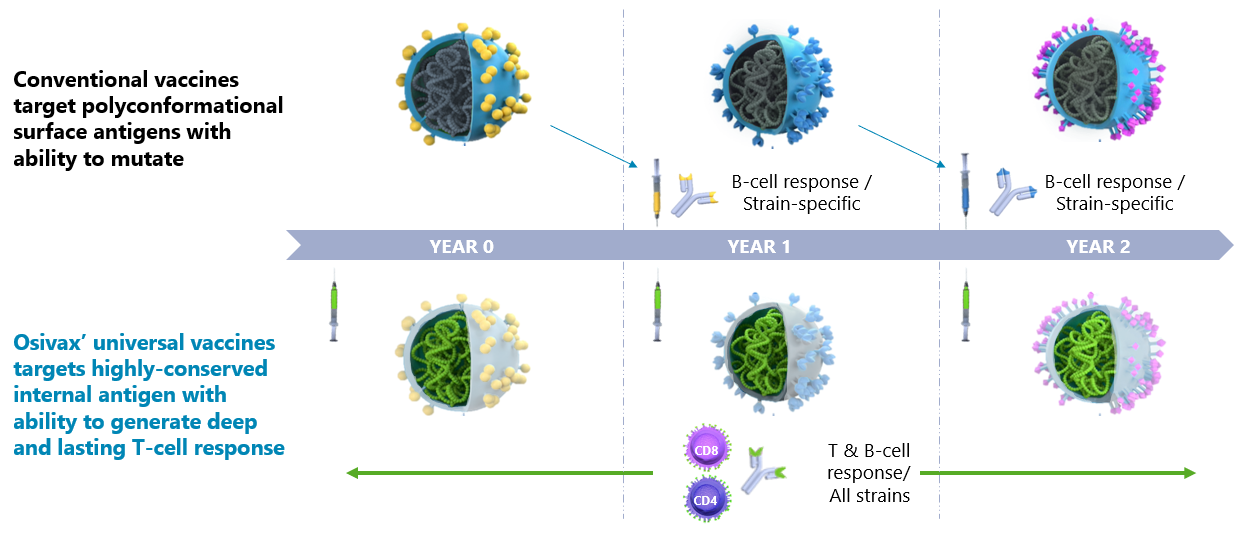
The innovative oligoDOMTM technology, an improved self-assembling Nanoparticles approach, targets internal and invariant parts of viruses in a very powerful way
References
Immunogenicity, safety, and preliminary efficacy evaluation of OVX836, a nucleoprotein-based universal influenza A vaccine candidate: a randomised, double-blind, placebo-controlled, phase 2a trial.
Leroux-Roels I, Willem P, Waerlop G, Janssens Y, Tourneur J, De Boever F, Bruhwyler J, Alhatemi A, Jacobs B, Nicolas F, Leroux-Roels G, Le Vert A.
THE LANCET Infectious Diseases, July 2023. doi: 10.1016/S1473-3099(23)00351-1
Safety and Immunogenicity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, Co-Administered with Fluarix® Tetra, a Seasonal Hemagglutinin-Based Vaccine.
Groth N, Bruhwyler J, Tourneur J, Piat E, Moris P, Le Vert A, Nicolas F.
Vaccines, May 2025. doi: 10.3390/vaccines13060558
Evaluation of Safety, Immunogenicity and Cross-Reactive Immunity of OVX836, a Nucleoprotein-Based Universal Influenza Vaccine, in Older Adults
Jacobs B, Leroux-Roels I, Bruhwyler J, Groth N, Waerlop G, Janssens Y, Tourneur J, De Boever F, Alhatemi A, Moris P, Le Vert A, Leroux-Roels G, Nicolas F.
Vaccines 2024, 12, 1391. doi: 10.3390/vaccines1212139
Randomized, Double-Blind, Reference-Controlled, Phase 2a Study Evaluating the Immunogenicity and Safety of OVX836, A Nucleoprotein-Based Influenza Vaccine.
Leroux-Roels I, Waerlop G, Tourneur J, De Boever F, Maes C, Bruhwyler J, Guyon-Gellin D, Moris P, Del Campo J, Willems P, Leroux-Roels G, Le Vert A, Nicolas F.
Front. Immunol., April 2022. doi: 10.3389/fimmu.2022.852904
Randomized Placebo-Controlled, Dose-Escalating Study to Evaluate OVX836, a Nucleoprotein-Based Influenza Vaccine: Intramuscular Results.
Withanage K, De Coster I, Cools N, Viviani S, Tourneur J, Chevandier M, Lambiel M, Willems P, Le Vert A, Nicolas F, Van Damme P.
The Journal of Infectious Diseases, Oct. 2021. doi: 10.1093/infdis/jiab532
OVX836 Heptameric Nucleoprotein Vaccine Generates Lung Tissue-Resident Memory CD8+ T-Cells for Cross-Protection Against Influenza.
Del Campo J, Bouley J, Chevandier M, Rousset C, Haller M, Indalecio A, Guyon-Gellin D, Le Vert A, Hill F, Djebali S, Leverrier Y, Marvel J, Combadière B and Nicolas F.
Front. Immunol., June 2021. doi: 10.3389/fimmu.2021.678483
OVX836 a recombinant nucleoprotein vaccine inducing cellular responses and protective efficacy against multiple influenza A subtypes.
Del Campo J, Pizzorno A, Djebali S, Bouley J, Haller M, Pérez-Vargas J, Lina B, Boivin G, Hamelin M-E, Nicolas F, Le Vert A, Leverrier Y, Rosa-Calatrava M, Marvel J, Hill F.
npj Vaccines, Jan. 2019. doi: 10.1038/s41541-019-0098-4.
OVX033, a nucleocapsid-based vaccine candidate provides broad-spectrum protection against SARS-CoV-2 variants in a hamster challenge model
Primard C, Monchâtre-Leroy E, Del Campo J, Valsesia S, Nikly E, Chevandier M, Boué F, Servat A, Wasniewski M, Picard-Meyer E, Courant T, Collin N, Salguero FJ, Le Vert A, Guyon-Gellin D, Nicolas F.
Front. Immunol., 19 June 2023. doi: 10.3389/fimmu.2023.1188605
OligoDOMTM: a T-cell response-enhancing platform applied to cancer immunotherapy
Del Campo J, Valsesia S, Nikly E, Ruiu R, Lacoviello A, Quaglino E, Cavallo F, Hannani D, Boucher E, Nicolas F, Le Vert A, Doro F.
Front. Immunol., 2025 Mar. doi: 10.3389/fimmu.2025.1549112
A safe and potentiated multi-type HPV L2-E7 nanoparticle vaccine with combined prophylactic and therapeutic activity.
Zhao X, Zhang Y, Trejo-Cerro O, Kaplan E, Li Z, Albertsboer F, El Hammiri N, Colaço Mariz F, Banks L, Ottonello S, Müller M
npj Vaccines, 2024 Jun. 9:119. doi: 10.1038/s41541-024-00914-z
Combined prophylactic and therapeutic immune responses against human papillomaviruses induced by a thioredoxin-based L2-E7 nanoparticle vaccine.
Zhao X, Yang F, Mariz F, Osen W, Bolchi A, Ottonello S, Müller M.
PLoS Pathog., 2020 Sep. e1008827. doi: 10.1371/journal.ppat.1008827
Minor capsid protein L2 polytope induces broad protection against oncogenic and mucosal human papillomaviruses.
Pouyanfard S, Spagnoli G, Bulli L, Balz K, Yang F, Odenwald C, Seitz H, Mariz FC, Bolchi A, Ottonello S, Müller M.
J Virol., 2018 Feb. 92:e01930-17. doi: 10.1128/JVI.01930-17.
Broadly neutralizing antiviral responses induced by a single-molecule HPV vaccine based on thermostable thioredoxin-L2 multiepitope nanoparticles.
Spagnoli G, Pouyanfard S, Cavazzini D, Canali E, Maggi S, Tommasino M, Bolchi A, Müller M, Ottonello S.
Sci Rep. 2017; 7: 18000. Published online 2017 Dec 21. doi: 10.1038/s41598-017-18177-1.
SnoopLigase peptide-peptide conjugation enables modular vaccine assembly
Andersson AC, Buldun CM, Pattinson DJ, Draper SJ, Howarth M.
Sci Rep. 2019 Mar 15;9(1):4625. doi: 10.1038/s41598-019-40985-w.
Combination of RTS,S and Pfs25-IMX313 Induces a Functional Antibody Response Against Malaria Infection and Transmission in Mice.
Brod F, Miura K, Taylor I, Li Y, Marini A, Salman AM, Spencer AJ, Long CA, Biswas S.
Front Immunol., 2018 Dec 4;9:2780. doi: 10.3389/fimmu.2018.02780.
Dual Plug-and-Display Synthetic Assembly Using Orthogonal Reactive Proteins for Twin Antigen Immunization.
Brune KD, Buldun CM, Li Y, Taylor IJ, Brod F, Biswas S, Howarth M.
Bioconjug Chem., 2017 May 17;28(5):1544-1551. doi: 10.1021/acs.bioconjchem.7b00174.
A HIV-Tat/C4-binding protein chimera encoded by a DNA vaccine is highly immunogenic and contains acute EcoHIV infection in mice.
Tomusange K, Wijesundara D, Gummow J, Garrod T, Li Y, Gray L, Churchill M, Grubor-Bauk B, Gowans EJ.
Sci Rep. 2016 Jun 30;6:29131. doi: 10.1038/srep29131.
Enhancing immunogenicity and transmission-blocking activity of malaria vaccines by fusing Pfs25 to IMX313 multimerization technology.
Li Y, Leneghan DB, Miura K, Brian IJ, Dicks MD, Fyfe AJ, Zakutansky SE, De Cassan S, Long CA, Draper SJ, Hill AV, Hill F, Biswas S.
Sci Rep. 2016 January 8;6:18848. doi: 10.1038/srep18848.
T Cell Responses Induced by Adenoviral Vectored Vaccines Can Be Adjuvanted by Fusion of Antigen to the Oligomerization Domain of C4b-Binding Protein.
Forbes EK, de Cassan SC, Llewellyn D, Biswas S, Goodman AL, Cottingham MG, Long CA, Pleass RJ, Hill AV, Hill F, Simon J. Draper.
PLoS ONE., 2012 September;7(9):e44943. doi:10.1371/journal.pone.0044943.
Fusion of the Mycobacterium tuberculosis antigen 85A to an oligomerization domain enhances its immunogenicity in both mice and non-human primates.
Spencer AJ, Hill F, Honeycutt JD, Cottingham MG, Bregu M, Rollier CS, Furze J, Draper SJ, Søgaard KC, Gilbert SC, Wyllie DH, Hill AVS.
PLoS ONE. 2012 March;7(3): e33555. doi:10.1371/journal.pone.0033555.
Immunocontraception in male feral swine treated with a recombinant gonadotropin-releasing hormone vaccine.
Campbell TA, Garcia MR, Miller LA, Ramirez MA, Long DB, Marchand JB, Hill F.
JSHAP., May 2010; 18(3):118-124.
The oligomerization domain of C4-binding protein (C4bp) acts as an adjuvant, and the fusion protein comprised of the 19-kilodalton merozoite surface protein 1 fused with the murine C4bp domain protects mice against malaria.
Ogun SA, Dumon-Seignovert L, Marchand JB, Holder AA, Hill F.
Infect Immun., 2008 Aug;76(8):3817-3823. doi:10.1128/IAI.01369-07.
Effective induction of high-titer antibodies by viral vector vaccines.
Draper SJ, Moore AC, Goodman AL, Long CA, Holder AA, Gilbert SC, Hill F, Hill AV.
Nat Med., 2008 Aug;14(8):819-821. doi: 10.1038/nm.1850.
